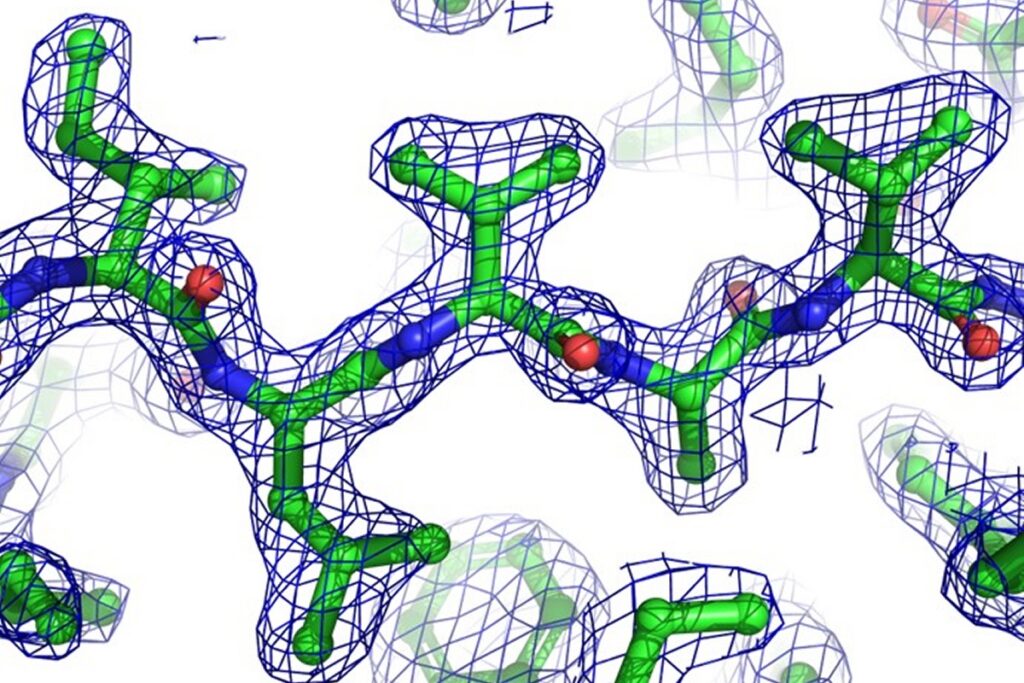
The X-ray crystallography is a widely used technique for determining the atomic and molecular structure of biological macromolecules like proteins, nucleic acids, and their complexes.
Shared Facility
https://www.med.upenn.edu/jf/bsbcore/centers/crystallography.html
Researchers
Kushol Gupta is a Research Assistant Professor in the Department of Biochemistry & Biophysics at the Perelman School of Medicine of The University of Pennsylvania, a member of the BMB graduate group, and directs the Johnson Foundation Structural Biology and Biophysics Core, a departmental resource that serves Penn and the greater region.
He is a structural biologist with expertise in both X-ray crystallography and solution biophysical methods, including small-angle X-ray and neutron scattering, light scattering, and analytical ultracentrifugation. His ongoing research focuses on retroviral integrases, their interaction with host factors, and a new class of drugs known as allosteric inhibitors of integrase (ALLINIs), which are potent antivirals against HIV. His research also includes other projects in the areas of phenylketonuria, RNA splicing, and site-specific recombination, highlighting the collaborative nature of research at Penn.
Publications
- Conformational plasticity of RAS Q61 family of neoepitopes results in distinct features for targeted recognition
- Structural principles of peptide-centric chimeric antigen receptor recognition guide therapeutic expansion
- Targeting of intracellular oncoproteins with peptide-centric CARs
- A Chicken Tapasin ortholog can chaperone empty HLA-B∗37:01 molecules independent of other peptide-loading components
- HLA3DB: comprehensive annotation of peptide/HLA complexes enables blind structure prediction of T cell epitopes
- Universal open MHC-I molecules for rapid peptide loading and enhanced complex stability across HLA allotypes
- Decoupling peptide binding from T cell receptor recognition with engineered chimeric MHC-I molecules
- Xeno interactions between MHC-I proteins and molecular chaperones enable ligand exchange on a broad repertoire of HLA allotypes








