CryoEM and CryoET
Cryo Electron Microscopy (cryoEM) has emerged as a revolutionary method in structural biology, enabling the determination of the three-dimensional structures of biological macromolecules at near-atomic resolution without the need for crystallization. We utilize cryoEM and cryoET to understand the structural mechanisms underlaying various fundamental or disease-causing biological processes.
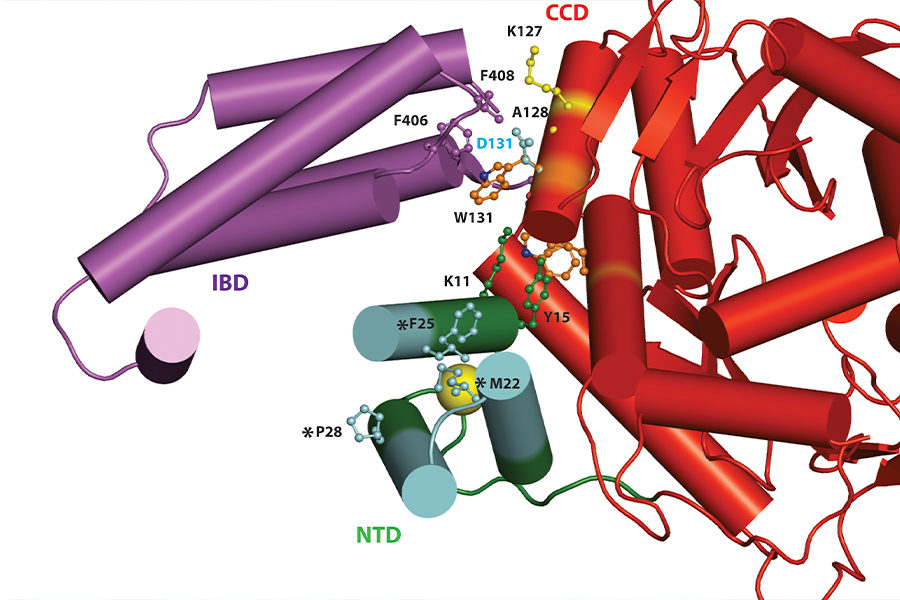
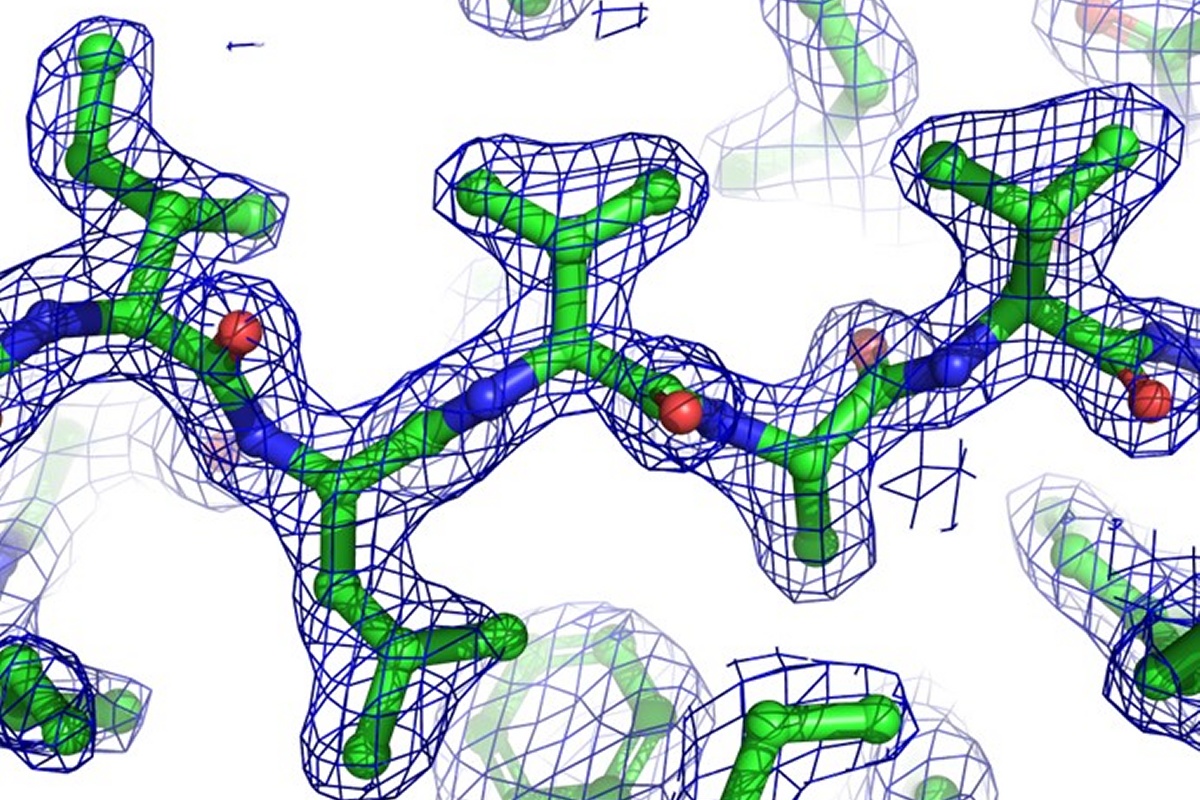
X-ray Crystallography and Biophysics
The X-ray crystallography is a widely used technique for determining the atomic and molecular structure of biological macromolecules like proteins, nucleic acids, and their complexes.
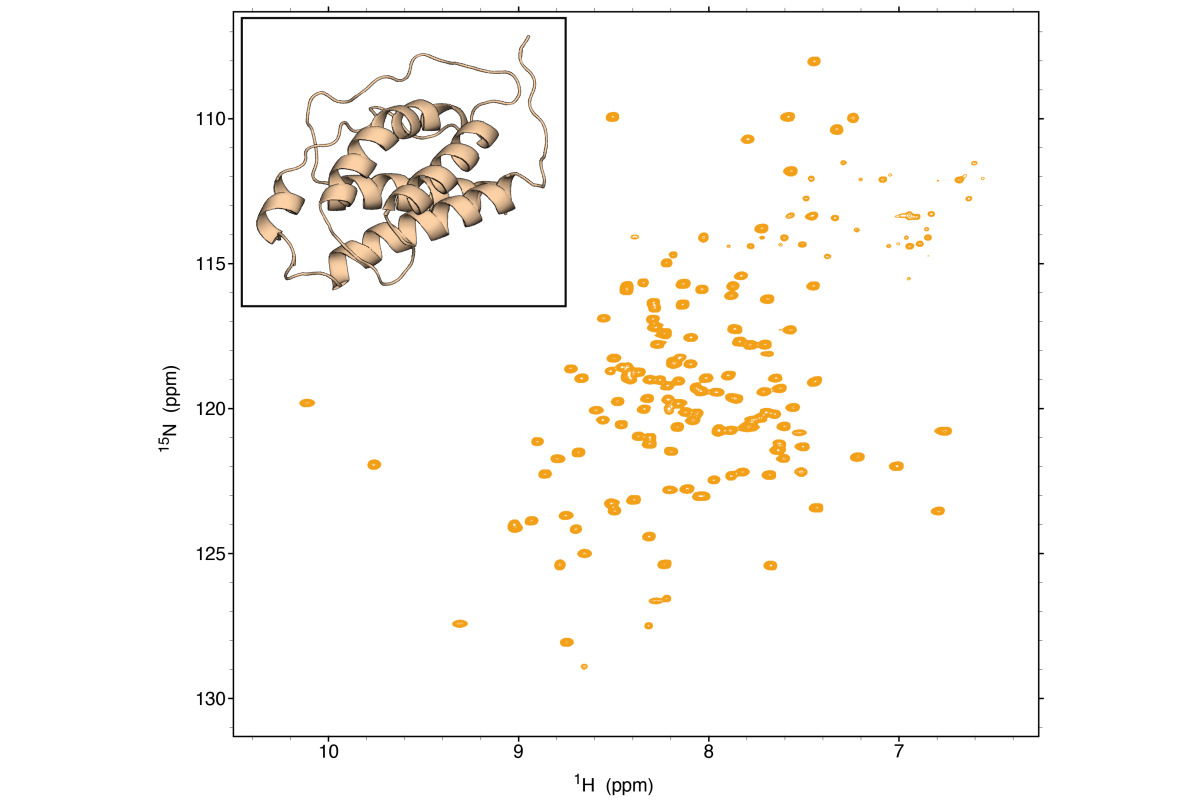
NMR
We utilize NMR spectroscopy for studying the structures, dynamics, and interactions of proteins and nucleic acids. NMR is essential technique for understanding protein-ligand interactions, protein-protein interactions, and conformational changes. NMR is also uniquely capable to elucidate structures and conformational dynamics of RNA, RNA-protein and RNA-small molecule interactions. Obtaining insights into complex interactions, characterizing biomolecular dynamics, and determining pathways for conformational exchange not only reveals insights into biological function, but provides molecular blueprints to develop new therapeutics.
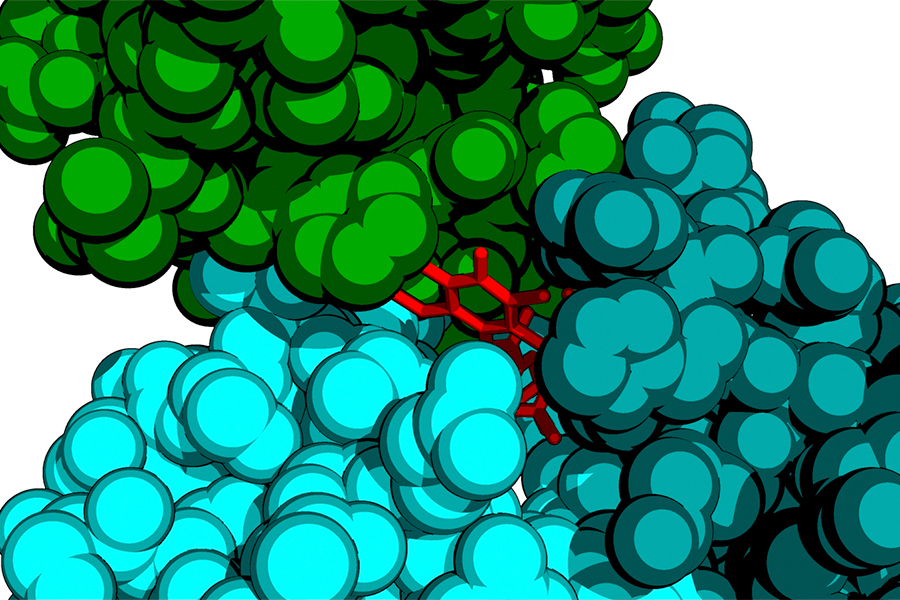
Computational Biology
We seek to understand complex biological processes and inform the development of new drugs and proteins based on computer models of biomolecules’ structure and dynamics. Major foci include using simulations to understand proteins’ moving parts and using deep learning to design new proteins and drugs.
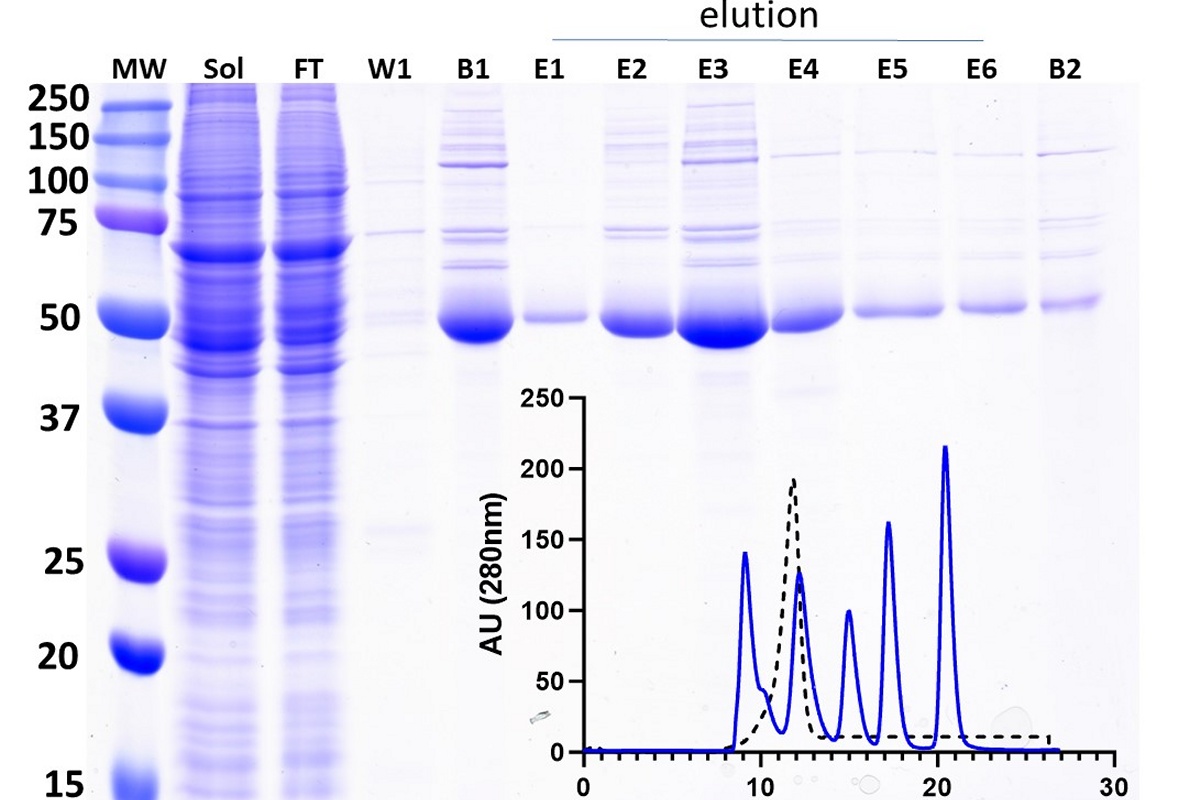
Protein Production Services
The HTSC Core provides scaled expression of recombinant proteins to the Penn research community. Proteins can be used for biophysical/biochemical characterization, crystalography, cryo-EM, and high-throughput screening to identify small molecule inhibitors. The HTSC Core offers services to express proteins in E. coli, baculovirus expression vector systems, and mammalian cells using the Expi293 system. These activities are provided through a centralized laboratory under the direction of an experienced Core Director who has a history of collaboration with investigators on protein expression projects. The Core supports all aspects of recombinant protein expression, including expression construct design, baculovirus preparation, and analytical/ preparative scale productions in all expression systems. The Core also provides protein purification services. Protocols developed by the core are used to purify recombinant proteins from soluble extracts or cell supernatants, using appropriate affinity matrices. When necessary, a second purification step (e.g., affinity, ion exchange, size exclusion chromatography, etc.) will be used to ensure maximum purity of the recombinant protein.
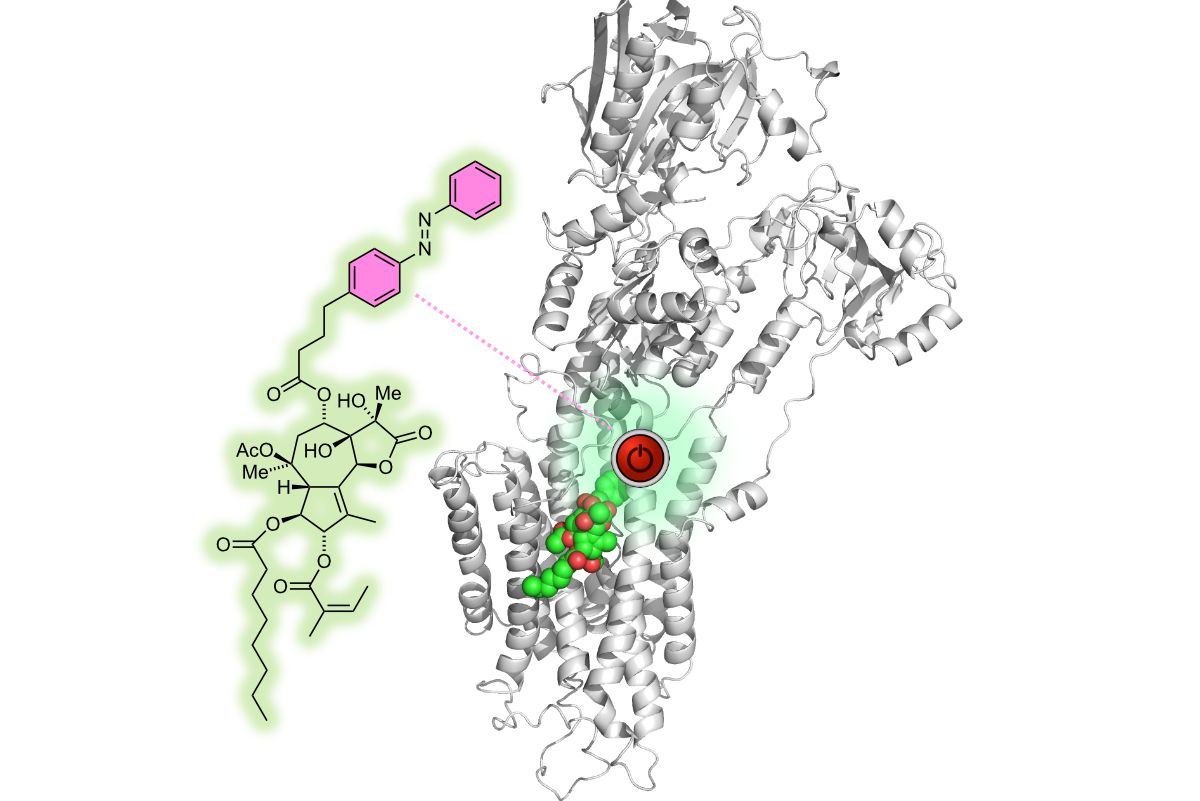
Chemical Biology
Chemical Biology uses chemical approaches to reveal new molecular insights into macromolecules and their complexes with proteins, nucleic acids and/or small molecule compounds, probe molecular and biological activities at the individual or systems level and develop new diagnostics and treatments for various diseases.
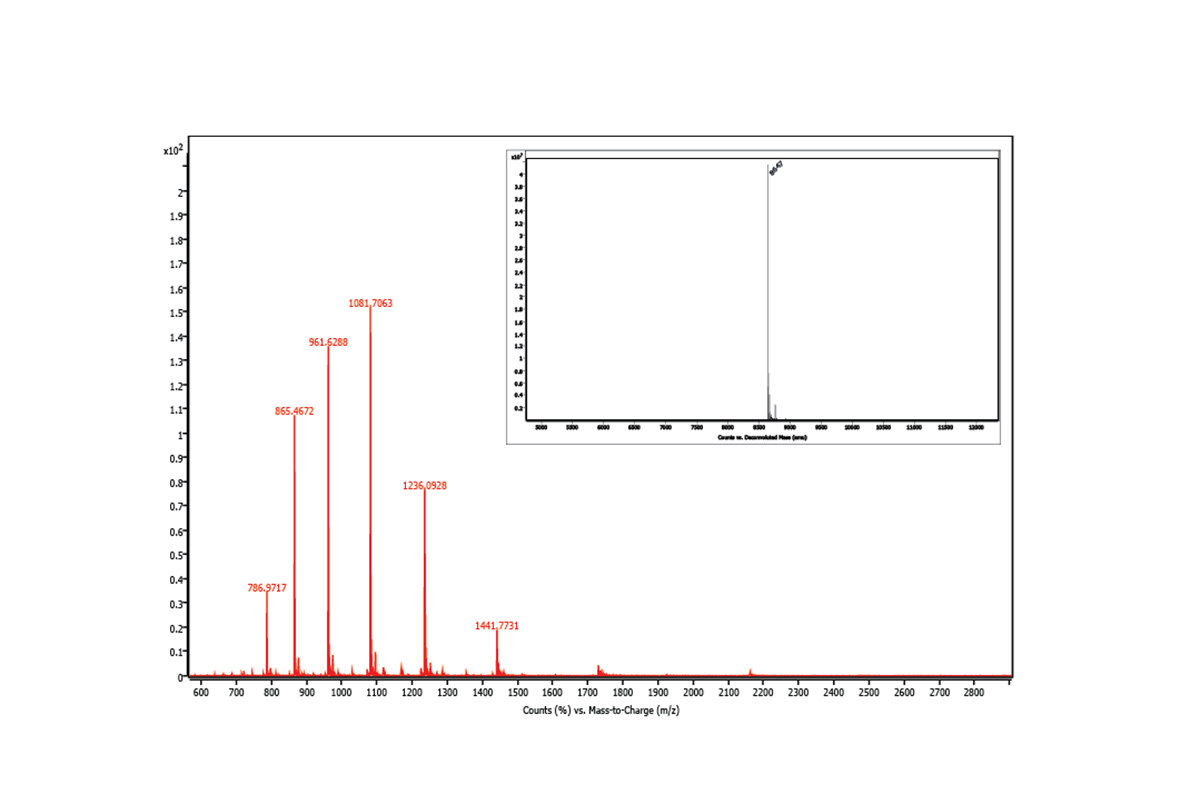
Mass Spectrometry
Mass spectrometry is an analytical technique that is used to measure the mass-to-charge ratio of ions, including peptides, proteins, small molecules, and nucleic acids. It can be used for a multitude of purposes, including the discovery of system-wide perturbations, the study of protein dynamics, and the chemical labeling of proteins with either endogenous or exogenous molecules.
Single Molecule Imaging
Single molecule techniques investigate the complex behaviors of biomolecules in vitro and in cellular environments, revealing their molecular structure, dynamics, interactions, and functions. By avoiding the need for averaging that is inherent in bulk measurements, these powerful methods provide nuanced insights into the diverse nature of molecular activities, enhancing our understanding of molecular heterogeneity.


